Description
Microbial induced corrosion (MIC) is the effect of the presence of microorganisms on the surface of a metal, resulting in the attack of the metal-microbe interface. The attack generally leads to rapid pitting corrosion.
There are various names applied to the phenomena, such as; microbial influenced corrosion, microbiological corrosion, biological corrosion etc.
The organisms that are known to cause attack include bacteria, algae, and fungi (e.g. yeasts and moulds), and by their very nature are present in most environments. An awareness of their effects is often limited at best, and many failures initially assumed to be associated with ‘just’ corrosion, may actually be initiated by microbial activity.
Some of the common bacteria cited in biological corrosion are:
- Desulfovibrio desulfuricans;
- Desulfotomaculum nigrificans (Clostridium);
- Delsulfomonas (The desulfo bacteria may often be grouped as sulphate reducing bacteria (SRB).);
- Thiobacillus ferro-oxidans;
- Gallionella;
- Pseudomonas aeruginosa.
This white paper only provides a broad view on the general mechanisms of MIC, and the reader is encouraged to explore the numerous texts and papers available on the subject.
Mechanism
The effects of the presence of microbes on the surface of a metal are numerous and complicated due to the different ways that the microbes can grow, and these may be affected by their nutritional requirements, exposure temperatures, anaerobic and aerobic environments.
Broadly, MIC may be considered to consist of three main stages:
- Creation of a biofilm;
- Change of environment at the metal surface;
- Deterioration of the metal.
Biofilms are used by bacteria to bind to the metal surface and multiple bacteria may be found within the same film, with the activity of one bacterium potentially affecting another. Biofilms may be found in the form of thin films or slime, through to larger mounds and tubercles. Initially, these will affect the surface potential such as by changes in the availability of oxygen (i.e. the change in the environment at the metal surface). Under the microbe colony, oxygen is reduced compared to the surrounding material and this generates an oxygen concentration cell. Areas low in oxygen are anodic compared to the areas high in oxygen availability, electrons flow from the anode to the cathode with metal ions being released resulting in deterioration (loss) of the metal at the anode.
The electrochemical process at the anode results in the reduction of pH. For example, the action of Thiobacillus, an aerobic sulphur-oxidising bacterium, can create an environment of up to 10% H2SO4 (sulphuric acid). The increased acidity contributes to attack of the underlying metal by dissolution. As the microbial activity increases, the rate of attack by pitting accelerates and other damaging species, such as chlorides, may be drawn into the location of attack as part of the electrochemical process, which again accelerates attack.
Appearance and Examples
Typical failures by MIC involve pitting attack of the material. By its nature, pitting may go unnoticed until it has progressed through the full thickness of the material and small ‘pin holes’ appear at the surface. As an example, the 316L stainless steel pipework of a plant that processed detergents started to exhibit pin-hole leaks from multiple locations, only a few weeks after installation. The pipes had been welded on site, installed, hydraulically tested for leaks using mains water, followed by a trial run of product through the pipes, flushed with acidified water, and then the system was isolated over a company shut-down period; on start-up, leaks were noticed. Examination of the inside of the pipes revealed multiple isolated pustules of corrosion deposits as shown below.
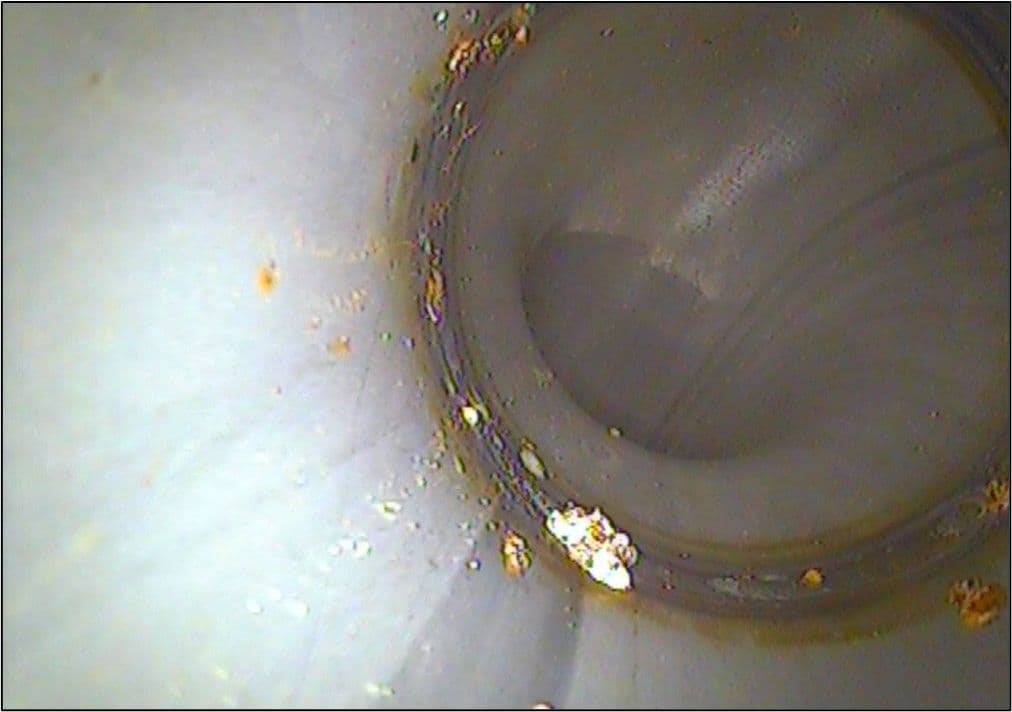
The leaks were primarily associated with the fabrication welds and the image clearly shows the condition of the weld root, exhibiting heavy oxidation at the weld and heat affected zone. Such oxidation severely reduces the corrosion resistance of stainless steels and would have rendered the material prone to corrosion attack anyway, although the same welding contractor had completed the installation of all the other pipework systems in the facility over some months without any problems having occurred. It was noted that the welds on the other systems also exhibited the same degree of oxidation and since these had been in operation for many months, and had not shown any signs of corrosion, it was considered by the client that their condition was acceptable for the application.
Microbes are known to be drawn to locate and grow at such features as weld heat tints/oxidation and the general characteristics of the pustules were typical of MIC. Additional pits, albeit at a much lower occurrence, were also observed on the seams of the pipes as shown in the image below and this demonstrated the propensity to attack of these features on the pipework. These seams, having been produced by the manufacturer of the pipe, were in a condition typical of that produced in pipe production, with a smooth surface finish and no significant oxidation with only a minor change in visual appearance, yet still they attracted attack.

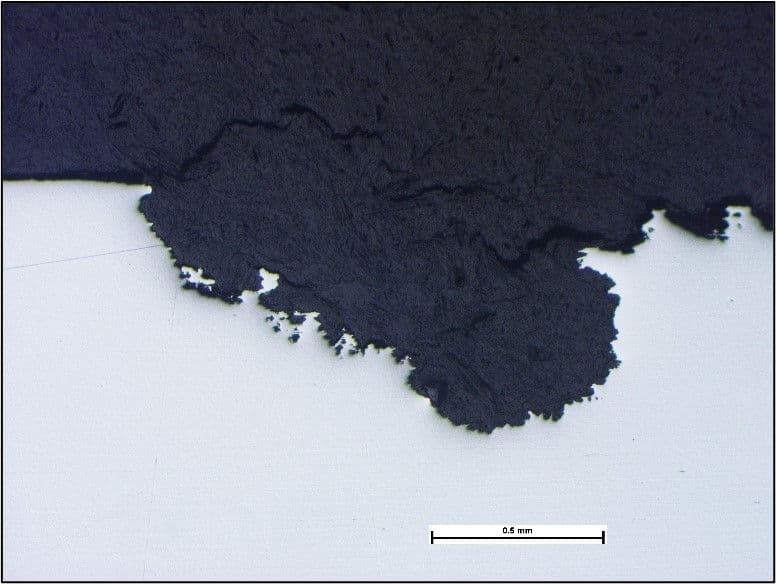
Laboratory examination revealed pitting attack beneath the pustules as expected. The image below shows one such area. The bright, light grey area surrounding the pits indicated that attack was still active.

A section through an area of attack revealed the typical features of pitting attack (below).
At the suggestion of MIC as being the likely cause, the client expressed surprise given that cleaning of the pipework had been undertaken with an acidic agent with the intent to kill microbes that could contaminate the product (they were conscious of the need to prevent microbial activity, but only with regard to product contamination). Their usual procedures were robust enough to prevent such contamination, but, in this case, not sufficient to prevent MIC. However, after review, their antimicrobial procedures were not undertaken until just prior to the test run of the product, and the system had therefore remained wet up until this point creating an ideal environment for microbes to develop. This demonstrates the potential for microbes to quickly develop a protective coating over the colony that can be impervious to chemicals, biocides, or other environments that could kill them so early action is critical. In addition, microbes may also congregate in crevices where they are relatively protected from ‘attack’ by biocides.
On investigation, it was found that the affected pipework differed from the other systems at the facility in that it was a CIP (clean in place) line with multiple ‘dead-ends’ creating areas of long-term stagnation which again were ideal for microbial activity. Due to the distribution of the pitting it was recommended that the entire system be replaced, with changes in the welding procedures to prevent oxidation at the weld roots, followed by chemical cleaning to first pickle and then passivate the inner surfaces. Any hydraulic tests on commissioning should also be completed with biocides and measures taken to prevent microbial activity.
Avoidance
As with most things, prevention is better than cure and the same can be said of MIC, but MIC is still a failure mechanism that surprises many engineers. Education then, may be the best form of prevention since with the awareness and knowledge, preventative measures can be adopted. However, the prime method of preventing MIC is the elimination of microbes, either by prevention of their ingress in the first place or early treatment before they have established colonies. Manufacturers of components and assemblies should have an awareness or understanding of the potential for MIC failures and incorporate measures for its prevention and biocides are a vital tool in this undertaking. In addition, hydrotesting should ideally be carried out with demineralised high purity water and the system dried at the earliest opportunity following draining, with measures undertaken to prevent moisture ingress. Furthermore, routine testing or condition monitoring of process or testing fluids could be carried out to check for the presence or levels of microbes to provide early warnings of activity.
So, the measures required to prevent microbial activity may be relatively straightforward and there are many propriety chemicals specifically developed for such treatment, and companies that specialise in the work, to support process engineers in combatting MIC.
