Description
The microstructure of a metal is made up of separate grains and, as its name suggests, intergranular corrosion is attack that is localised along or adjacent to the boundaries of these grains.
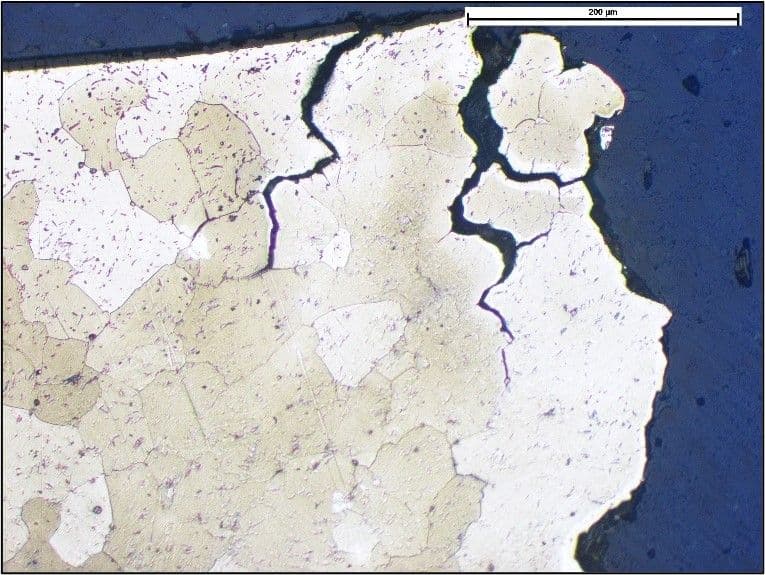
Intergranular corrosion attack of an aluminium alloy.
The attack can result in cracks that penetrate deep into the material, and is often associated with localised changes in the material composition at these boundaries.
With intergranular corrosion, the grain boundary is the preferred route of attack because of:
- The mismatch in the atomic structure that is inherent at the boundaries between the ordered atomic structure of the grains, creating a suitable path for corrosion;
- Compositional variations between the bulk of the grain and these boundaries.
An alloy is a mix of two or more elements and when a metal alloy solidifies from the molten stage, crystals (grains) grow, developing a shell of solidifying metal with a constantly changing composition, dependant on the particular alloy. The elements or portions of the melt that contain lower melting point phases or elements are pushed out ahead of the solidifying grains until the surfaces of adjacent grains meet; these form the grain boundaries. These boundaries can then exhibit a variety of elements and phases, some of which can be deleterious to the material and which may render these boundaries more susceptible to corrosion attack.
In addition to impurities, an alloy may be susceptible to intergranular corrosion due to the presence of secondary phases at the boundaries. These phases could be either normal, or unintended, for the particular alloy system. For example, aluminium alloys can develop multiple phases, that are normal and an inherent part of the structure. Some of these may be anodic to the matrix phase and then corrode in preference to the matrix. A preferred distribution of these phases would be equally spaced throughout the microstructure, but processing or heat treatments may cause these phases to precipitate out and concentrate at the grain boundaries, rendering these boundaries more susceptible to attack than the surrounding matrix. Alternatively, unintended phases may be produced because of incorrect heat treatment or exposure to thermal ‘damage’, such as the sensitisation of stainless steels from welding, or exposure to high temperatures during service, producing chromium-carbides at the grain boundaries. In the formation of the carbides, chromium is drawn out of the surrounding matrix, and since chromium is the main element that causes these steels to be ‘stainless’ (or more correctly, corrosion resistant) i.e. resistant to corrosion, the area denuded of chromium is then less resistant to corrosion and attack can then proceed along these grain boundaries. Austenitic stainless steels intended for welding or exposure to high temperatures should then be of the low carbon grades (i.e.<0.03%C) or grades ‘stabilised’ with niobium or titanium. These two elements combine with carbon in the metal in preference to the chromium, preventing the formation of chromium-carbides.
Appearance
Intergranular corrosion may be difficult to identify just by examination of the affected surface alone, and may only be indicated by light discolouration, staining, minor corrosion, or oxidation. For example, an aluminium alloy component used for a fixture in an automotive application had fractured in a brittle manner and examination of the surface only revealed very light corrosion deposits. However, microexamination of a cross-section through the component revealed extensive intergranular corrosion as shown below.
In some highly worked materials, such as with wrought or forged aluminium, intergranular corrosion attack may extend along the deformed grain boundaries and this will appear as layers or flakes of metal interspersed with oxide products. This is often termed as exfoliation corrosion. As attack continues, the corrosion oxides expand, leading to swelling of the material, and flaking of the metal surface or coatings.
Avoiding
Avoidance of intergranular corrosion will depend on the underlying cause but may include the flowing considerations: use of materials with a greater resistance to such attack, for example, by use of higher alloyed materials; isolation of the material from the environment by the use of coatings, or other ‘protective layers’, such as overlays of weld metal with greater resistance to attack; control of the environment (if possible) to make it less corrosive; correct heat treatment to remove deleterious microstructural phases.
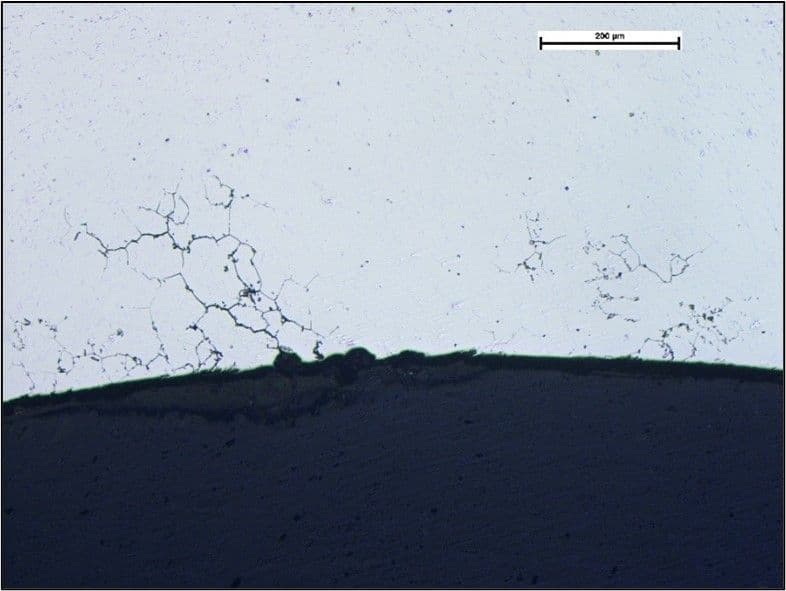
An example of intergranular corrosion that could have been avoided is shown in the image below, taken at X500 magnification.
This shows the inner surface of a 316L stainless steel tube. The tube had been stored for some weeks and just prior to its use in service, a light stain was observed on the inner surface, and high magnification examination revealed grain boundary attack. Metallurgical examination revealed that the surfaces of the tube had been sensitised as evidenced by the presence of chromium-carbide precipitates at the grain boundaries, for a depth of approximately 30µm. Carbides were not observed through the tube section remote from the surfaces, as would be expected for a low carbon grade, and it was concluded that carbon diffusion had occurred during manufacture, probably during heat treatment. Analysis of the surface by scanning electron microscopy with associated energy dispersive x-ray techniques (SEM-EDX) revealed high levels of chlorine and sodium. These were probably in the form of ‘salt’ (sodium-chloride) and the chlorine ion, chloride, is known to readily attack stainless steels and was the probable cause of the intergranular corrosion observed.
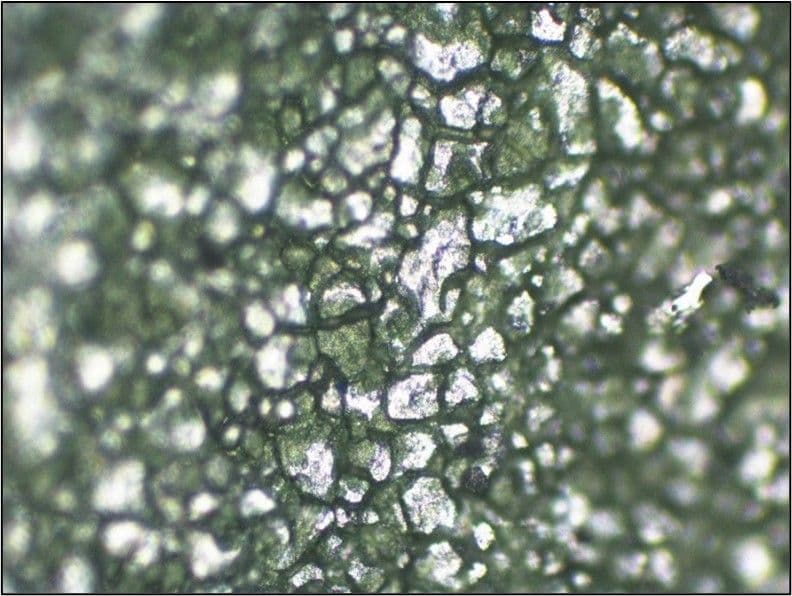
Aluminium alloys may be prone to attack in alkali environments. An aluminium compressor wheel used in a turbo charger had suffered a catastrophic failure. Examination revealed isolated areas of corrosion attack, deposits high in chlorine and sodium (probably from contaminated air being drawn into the engine), in addition to intergranular attack at the area of crack initiation, as shown in the image below taken using the SEM. The intergranular corrosion attack caused high stress concentrations leading to the rapid fatigue fracture of the wheel.

