Description
Atomic hydrogen is the smallest element in the periodic table of elements, yet its effects on metals can be catastrophic. Its detrimental effects on iron were first remarked upon in 1875 by W.H. Johnson, in a paper titled, ‘On some remarkable Changes produced in Iron and Steel by the Action of Hydrogen and acids’[1].
Metals are made up of atoms arranged in crystal structures or lattices, and hydrogen, being small, can readily migrate through the crystal lattice. Its effect on the metal is primarily one of reducing ductility (i.e. increasing a propensity to behave in a brittle manner) and load-bearing capacity, causing cracking at stresses below the yield stress, but also the formation of internal defects, hardening the metal by solid solution strengthening, and in certain metals such as titanium, the formation of hydrides leading to embrittlement.
The term Hydrogen Embrittlement (HE) can be considered as an umbrella term covering a multitude of mechanisms or outcomes relating to the degradation or failure of materials. This white paper focuses on the form of HE referred to as Hydrogen Induced Cracking (HIC).
Mechanism
Hydrogen is an abundant element and readily available, and particularly from water inherent in most environments. Hydrogen can enter a steel during the steel-making process, welding, chemical cleaning, plating, or as a product of corrosion processes.
With HIC, the hydrogen atoms in the metal are attracted to areas of high stress or voids in the metal, which then combine into hydrogen molecules (H2). As more molecules combine, a gas is formed with a resulting increase in pressure, which can reach a point where the internal gas pressure generates stress in the metal that is greater than the material’s tensile strength, and cracks are created. The orientation of these cracks may be influenced by the microstructure such as along the grain flow in forged components or plates, or by tensile stress, either residual or applied, with cracking orientated perpendicular to the principle stress direction.
Appearance
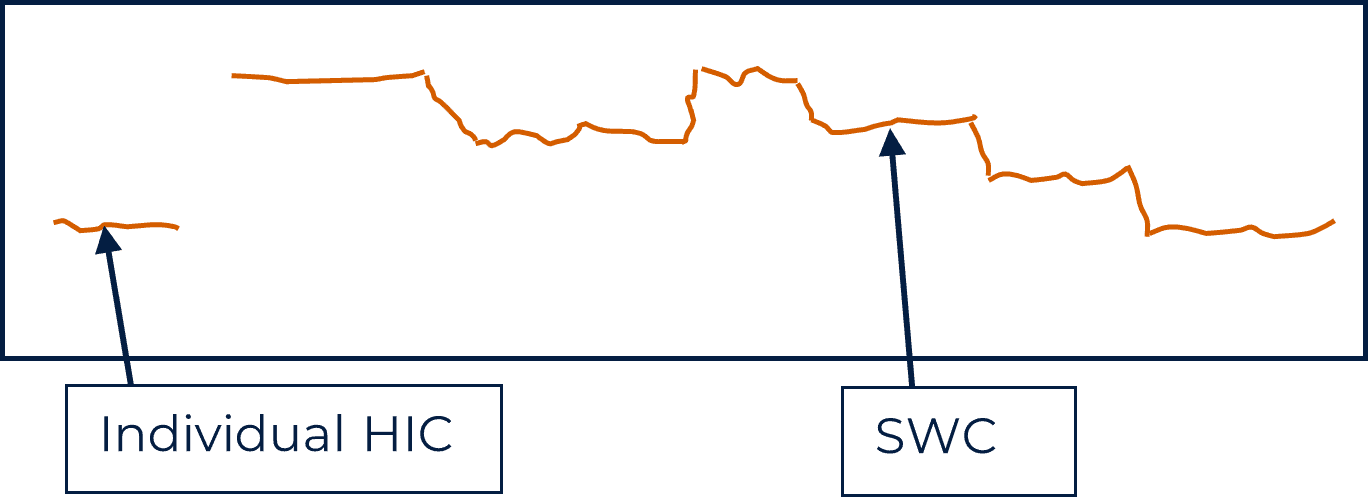
Unfortunately, by its nature HE may go unidentified until fracture occurs but HIC may manifest as blistering of the surface. These are caused by cracking close to the surface of the material. Cracks deeper into the material will remain hidden and their identification would require inspection by non-destructive examination. Cross-sections through affected material would reveal cracking orientated in the direction of grain flow and the cracks may be long and straight, or stepped. Stepped, or stepwise cracking (SWC) is formed when multiple parallel cracks that are close to each other, join by fracture across the intervening ligaments of metal as shown in the diagram below.
Avoiding Hydrogen Induced Cracking
As the processes involved in HE and HIC are complex and varied, the measures to avoid HE will also be varied. Such measures may include the following;
- Use of clean steel to avoid the occurrence of inclusions or voids;
- Use of coatings to prevent the ingress of hydrogen from the environment or corrosion processes;
- Use of inhibitors in the surrounding solution to prevent corrosion;
- Elimination of poisons that prevent the formation of molecular hydrogen, leaving atomic hydrogen that can diffuse into the material;
- Use of different materials that exhibit resistance to HIC based on their composition or hardness; a commonly cited maximum hardness for materials to avoid HIC is 22HRC;
- Reduce corrosion rates with cathodic or anodic protection;
- Baking to remove hydrogen;
- Maintenance of tight controls on manufacturing and plating to avoid evolution of hydrogen;
- Correct storage of welding consumables and welding in appropriate atmospheres e.g. not wet or damp.
