Description
Atomic hydrogen is the smallest element in the periodic table of elements, yet its effects on metals can be catastrophic. Its detrimental effects on iron were first remarked upon in 1875 by W.H. Johnson, in a paper titled, ‘On some remarkable Changes produced in Iron and Steel by the Action of Hydrogen and acids’[1].
Metals are made up of atoms arranged in crystal structures or lattices and hydrogen, being small, can readily migrate through the crystal lattice. Its effect on the metal is primarily one of reducing ductility (i.e. increasing a propensity to behave in a brittle manner) and load-bearing capacity, causing cracking at stresses below the yield stress, but also the formation of internal defects, hardening the metal by solid solution strengthening, and in certain metals such as titanium, the formation of hydrides leading to embrittlement. The effects of hydride embrittlement will be discussed in another white paper.
The term Hydrogen Embrittlement (HE) can be considered as an umbrella term covering a multitude of mechanisms or outcomes relating to the degradation or failure of materials. Research continues into the different forms of HE as it is still not fully understood. As a consequence, there are many different descriptions and terms applied to these forms, and often, confusingly, different terms may apply to the same general mechanism.
HE does not affect all metals equally and high-strength steels, aluminium alloys and titanium alloys are the most severely affected. This current paper will only be concerned with the more typically encountered forms of HE in steel and will use some of the more common terminology.
Mechanism
Hydrogen is an abundant element and readily available, and particularly from water inherent in most environments. Hydrogen can enter a steel during the steel-making process, welding, chemical cleaning, plating, or as a product of corrosion processes. Its source may then be considered as either, 1) a bulk effect, in that it is inherent (internal) in the metal from production, from processing such as welding, or 2) the material surface (external) such as that derived from corrosion processes.
The main processes involving bulk or internal hydrogen are;
- Hydrogen Induced Cracking (HIC)
- Stress Orientated Hydrogen Induced Cracking (SOHIC)
- Step Wise Cracking (SWC)
These processes lead to the development of internal cracks which, if close to a surface, can appear as blisters.
The hydrogen atoms in the metal are attracted to areas of high stress or voids in the metal, which then combine into hydrogen molecules (H2). As more molecules combine, a gas is formed with a resulting increase in pressure, which can reach a point where the internal gas pressure generates stress in the metal that is greater than the material’s tensile strength, and cracks are created. The orientation of these cracks may be influenced by the microstructure such as along the grain flow in forged components or plates, or by tensile stress, either residual or applied, with cracking orientated perpendicular to the principle stress direction.
The main processes involving externally sourced hydrogen are;
- Hydrogen Stress Cracking (HSC)
- Sulphide Stress Corrosion Cracking (SSCC)
- Stress Corrosion Cracking (SCC), associated with HE, and some sources consider these mechanisms to be synonymous with each other. However, SCC will be discussed in a separate white paper for now.
Appearance
Unfortunately, by its nature HE may go unidentified until fracture occurs. A common example of HE failures is that of high strength fasteners that have fractured catastrophically (instantaneously) either hours or days following installation, and particularly so with galvanised fasteners.
Fasteners that have failed in this way will generally exhibit a very brittle fracture. Tensile overload would be expected to exhibit some level of macro-ductility on the fracture face. Examination at high magnification by methods such as scanning electron microscopy (SEM) may be required to fully characterise the fracture. Tell-tale signs of HE-induced failure may include intergranular cracking as shown in the image below.
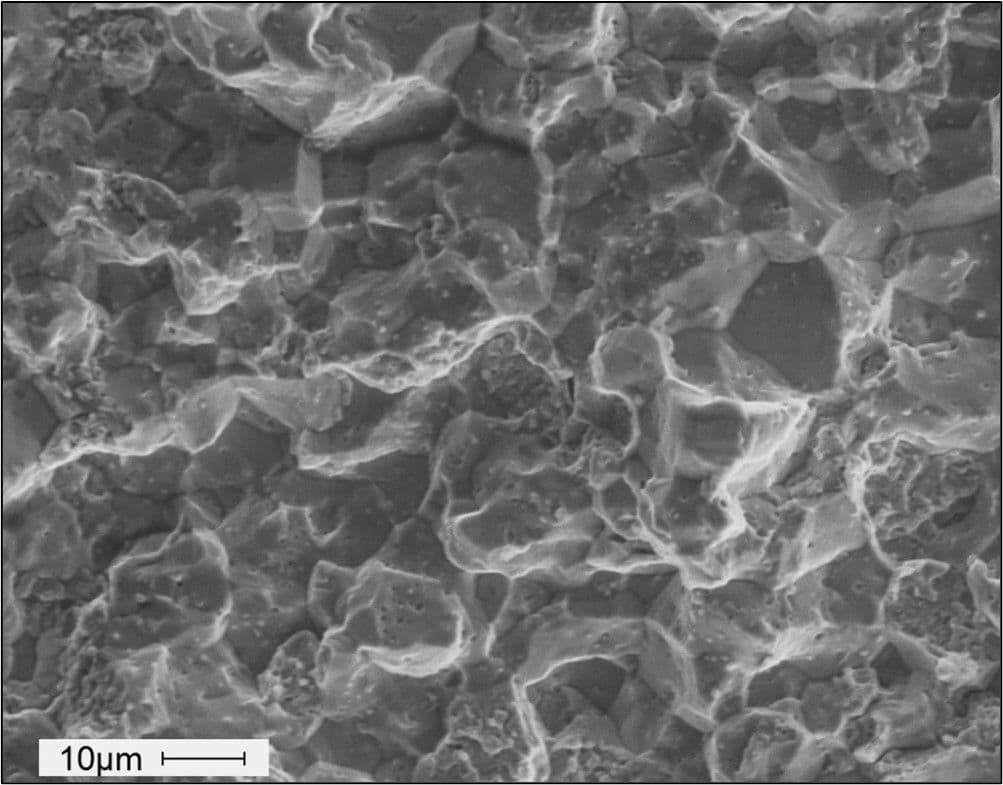
SEM image of the surface of a fractured bolt showing an intergranular fracture mode.
However, it is readily apparent that fracture surfaces may also exhibit evidence of cleavage, quasi- cleavage, and microvoid coalescence, complicating investigations into the cause of service failures and so it can be difficult to differentiate between HE, SCC, and tempered martensite embrittlement. Clearly, even with the use of an SEM, determining the cause of failure as HE can be problematic and also requires gathering circumstantial evidence, such as:
- If coatings such as zinc having been applied;
- Timing of fracture (delayed, within hours or days);
- Brittle fracture in a material that would normally exhibit some ductility;
- The population of fractures in a particular batch, with a high percentage of failures being indicative of a manufacturing problem such as poorly controlled plating procedures.
With regard to HSC and SSCC, being surface induced, cracks will generally develop at or close to the surface and may be identified using surface non-destructive testing techniques such as magnetic particle inspection or dye penetrant inspection. Cracking may propagate at fast rates and so if there is an awareness of the potential for cracking caused by hydrogen damage, a sufficiently frequent periodicity for inspection should be selected, but by its nature, predicting the onset of cracking may be particularly difficult at best.
A frequently asked question when HE is suspected, is whether analysis of the material would reveal high levels of hydrogen. Unfortunately, the levels of hydrogen in the material are generally so low when considering bulk analysis, as it is the concentration of hydrogen at localised points, rather than the overall content that is the problem. Also, once a fracture has occurred, hydrogen is immediately released, and the evidence is lost.
If, following a number of failures suspected of being caused by HE, a series of tests could be conducted to investigate if hydrogen is an issue e.g. testing to BSEN ISO15330. Tests generally utilise static tensile loads on samples in the as-received/manufactured condition and following various baking heat treatments. These heat treatments are carried out at low temperatures from 180°C to 220°C for periods between two and twenty-four hours. At these temperatures, hydrogen is readily mobilised and driven out of the material which would then be identified in the static load tests. Indeed, fasteners or other components may often be subjected to such baking processes at manufacture if there has been a risk of the ingress of hydrogen.
Avoiding Hydrogen Embrittlement
As the processes are complex and varied, the measures to avoid HE will also be varied. Such measures may include the following;
- Avoiding corrosion;
- Baking, as described above;
- Maintenance of tight controls on manufacturing and plating to avoid evolution of hydrogen;
- Correct storage of welding consumables and welding in appropriate atmospheres e.g. not wet or damp,
- Selection of materials, such as fasteners, of a lower strength, which are less susceptible to HE but which still provide adequate strength for the application; a commonly cited maximum hardness for materials to avoid HE is 320HV.
