Description
Galvanic, or bimetallic corrosion is a type of attack that occurs if two dissimilar metals are in electrical contact, and when in the presence of an electrolyte.
The effect of an electrical force that is developed when two dissimilar metals are in contact was identified by Luigi Galvan in the eighteenth century, who found that a frog’s leg moved when the exposed muscles and nerves were placed in contact with a bimetallic strip.
Mechanism
When two metals are placed in contact, an electrochemical cell can be created which results in the flow of electrons from the anode to the cathode. The process at the anode is one of oxidation (corrosion), which is of lower electrical potential, and reduction at the cathode, which is of higher electrical potential. In these systems, the cathode is generally protected from corrosion of the metal although it may experience a build-up of metallic deposits.
Different materials exhibit different electrical potentials and larger differences in potential will generate increased flow of electrons. Metals may be placed in order of potential in specific media (the electrolyte) and an often-cited order is that for exposure to sea water. Magnesium and zinc are at the more active, or anodic end, whilst platinum and gold are at the passive, or cathodic end of the series.
It may be observed that a particular metal may behave in either an anodic or cathodic manner, depending on what it is connected to. For example, copper in contact with titanium or nickel alloys will be anodic and will corrode. If the copper is in contact with low carbon steel, the steel will be anodic and corrode in preference to the copper, which is then cathodic.
At the surface of the anode a metal atom is ionised by release of an electron into the metal, forming a cation and the cation is released into the electrolyte; this is the corrosion mechanism resulting in the loss of metal. The electron then flows through the metal to the cathode to combine with other cations in the electrolyte. The rate of flow of electrons must be matched by reaction of the electrons with cations at the cathode and so if there are any restrictions in cation availability, the flow of electrons, and hence corrosion will also be reduced.
The relative areas of exposed metals also have a strong effect on the rates of corrosion. A large cathodic area relative to the anode will cause rapid rates of corrosion of the anode. Correspondingly, a small cathodic and large anodic area will only cause minimal or negligible corrosion of the anode.
Appearance and Example
Galvanic corrosion may be identified by the observation of highly localised attack of one of the two dissimilar metals in contact (i.e. the anode) and corrosion will typically occur close to the point where the two metals are in contact. Corrosion deposits, staining, or damage to coatings such as blistering of paint, may be tell-tale signs that galvanic corrosion is occurring.
The photomicrograph below shows the preferential corrosion attack of a steel component with a copper coating. The coating was damaged, exposing the underlying steel, which then corroded preferentially.
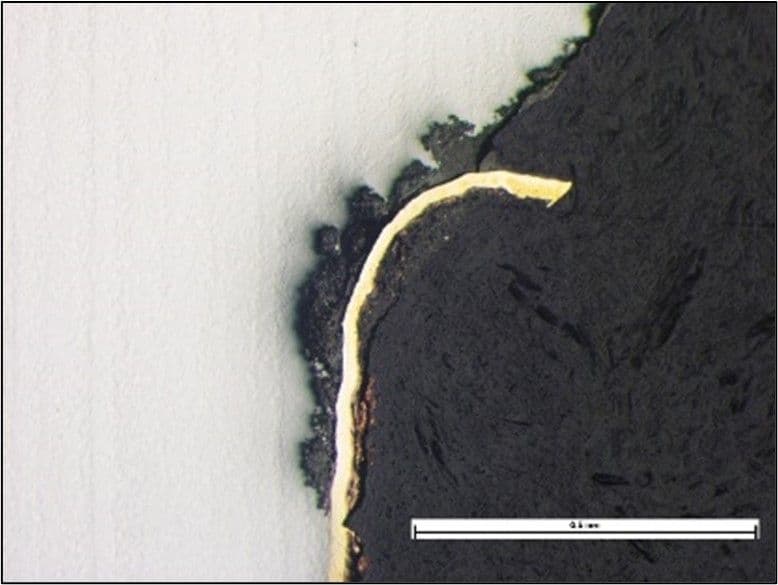
An example of galvanic corrosion is associated with chromium plating on steel. It may appear that the plating will protect the steel from corrosion, but any small breaches in the chromium will cause accelerated corrosion attack of the steel at the breach as the steel is anodic to the chromium. Furthermore, since the relative areas differ significantly, the large cathodic area of the chromium will drive rapid corrosion of the anodic steel, leading to severe pitting attack.
An early reported case of galvanic corrosion occurred on the Royal Navy ship HMS Alarm. In 1761, the hull of the vessel was clad in copper sheet below the water line to protect the wooden hull from the build-up of weed and attack from sea life. The sheets were attached to the hull with iron nails and after a voyage, some of the nails had ‘dissolved’ causing detachment of copper sheets. This is again, an example of a large cathodic area (the copper sheets) and a small anodic area (the nails).
Avoidance
There are a number of things that can be done to reduce, avoid, or mitigate against galvanic corrosion and each deals with one or more of the factors that cause it, namely, the flow of electrons between the dissimilar metals, the requirement for the use of dissimilar metals, and the presence of the electrolyte.
The flow of electrons may be prevented by breaking the electrical contact between the metals. This may be achieved with the use of insulators in the form of gaskets or coatings for example. Coatings must be kept intact though as any damage or breaches may allow electrons to flow and corrosion to commence. In the example of HMS Alarm, it was found that some of the nails used to secure the copper sheets had not been dissolved and the remnants of the paper used to wrap the sheets on delivery to the dockyard were observed to be trapped between the nail and the copper i.e. the sheets and nails were insulated from each other and at the time, it was concluded that iron should therefore not be allowed direct contact with copper in sea water.
At the design stage, it may also be possible to select materials that have a reduced potential difference, or even use of the same material if possible. Clearly, different materials have their uses, and this is why they are selected for a given task, but in the form of an assembly, the variety of metals may exhibit corrosion problems that outweigh the benefits of different materials to the individual components. Again, using the example of HMS Alarm, in 1783, it was decreed that iron nails or bolts should not be used to affix the copper sheets, but rather, copper or zinc bolts be used i.e. the potential difference reduced or eliminated. The strength of copper or zinc is clearly less than iron, but the reduction in strength could be accommodated, without being of detriment to the attachment of the copper sheets.
With respect to the presence of an electrolyte, this may potentially be eliminated by control of the environment, and corrosion can be stopped e.g. prevention of the build-up of moisture in the form of condensation. However, if a liquid electrolyte is an inherent part of the environment, then chemical additives may need to be added, which can inhibit the anodic, cathodic, or both reactions, and hence inhibit corrosion.
The Galvanic effect may be used to advantage, and this effect is utilised to protect steel structures such as ships, oil platforms, and pipelines. Large blocks of metal such as zinc may be bolted to the structure, such as the underside of a ship and these blocks corrode in preference to the hull as they are anodic to the steel; the zinc blocks being termed Sacrificial Anodes. Since this makes the hull cathodic in comparison to the zinc, this galvanic effect may be considered as a form of Cathodic Protection. More typically, cathodic protection is undertaken with the use of impressed potential difference/voltages. With impressed voltages, tight control must be maintained to balance the flow of electrons as cathodic reactions, left unrestricted i.e. ‘over-protection’, may produce atomic hydrogen, leading to hydrogen embrittlement of the steel.
