Description
As the name suggests, this is corrosion that occurs in a small gap, joint, or confined space; a crevice. It occurs as a consequence of water or some other working liquid being drawn into or residing in the crevice creating a corrosive environment, in a similar way to pitting corrosion. Crevices may be formed in a multitude of locations such as under loose coatings, seals and gaskets, fasteners and washers, any small gap, and under debris.
Materials that rely on a stable oxide layer to protect the underlying metal from further oxidation or corrosion are particularly susceptible to crevice corrosion e.g. stainless steels and aluminium alloys.
Crevice corrosion damage on a stainless steel surface in contact with a poorly bonded adhesive.
Mechanism
For the surface of a metal to resist ongoing corrosion, ideally a passive layer (typically an oxide) forms and which subsequently resists further oxidation or corrosion, or at least reduces it to a small and/or ‘acceptable’ level with respect to service performance and life. However, if an area on the surface is affected by damage or chemical attack and is depassivated (i.e. is no longer passive, and is ‘active’ for further oxidation), it becomes anodic to the remainder of the surface, which is cathodic. If it cannot immediately repassivate as would happen in the presence of sufficient oxygen, the affected surface is then prone to continued corrosion attack.
Many environments contain small levels of potentially damaging species such as chlorides, but the low levels do not exhibit a risk of corrosion to the metal in a bulk environment. A crevice, however, will draw moisture or liquids into it which then become stagnant, or which allow for the concentration of the damaging species to levels that can then cause corrosion.
The stagnant conditions within a crevice can promote corrosion due to the variation in oxygen content. The low levels within the crevice become anodic relative to the higher oxygen levels at the edge of the crevice, which are cathodic. General attack of the surfaces remote from the crevice may self-repair but in the crevice, this may be prevented due to the low levels of oxygen.
The anode-cathode couple that forms in a crevice generates an electrical circuit at a micro-scale with the flow of electrons from the anode to the cathode through the metal with a corresponding release of positively charged metal ions into the electrolyte (liquid) from the anode (crevice). The release of these metal ions is the effective loss in metal from the crevice which causes it to increase in size.
The energy in this electrical circuit is increased with the relatively small area of anode compared to the large cathodic area. This drives the crevice corrosion process in a similar way to pitting corrosion.
The positively charged metal ions released in the crevice also attract any negative ions in the liquid environment, such as chlorides, which are aggressive with respect to corrosion and can drive the crevice corrosion process.
Appearance
Crevice corrosion may often go unnoticed until a more significant failure occurs such as the loss in integrity of a joint, or the development of secondary failure mechanisms that initiate from crevice corrosion. For example, stress, combined with corrosion in the crevice can lead to stress corrosion cracking (SCC); a cracking mechanism that can rapidly lead to catastrophic fracture of a component.
Furthermore, crevice corrosion may only be identified by discolouration or staining permeating from the crevice, blistering of paint and other similar coatings, or the build-up of corrosion debris at the edge or opening of the crevice.
On an affected surface, crevice corrosion is typically manifest as shallow patches of corrosion attack, occasionally with minimal corrosion deposits, which, as described above, will often be present on the edge of the crevice or somewhere remote from it.
Avoiding Crevice Corrosion
Understanding the potential risk to a material that crevices present, should assist the designer or maintenance engineer for example, to specify processes or measures to avoid the formation of a crevice in the first place. Such measures may include the following;
- Use of coatings or sealants at joints, ensuring that they do not degrade to a point where a crevice develops,
- Design to avoid prolonged exposure to liquids or allow for adequate drying,
- If possible, use methods of joining components such as welding, to avoid a crevice,
- Correct storage and handling of materials,
- Routine inspection and regular reapplication of compounds that may degrade with time, such as greases in joints and at fasteners,
Examples
Example One
As an example of the second and perhaps fourth bullet points above, the stainless steel guard rails on a luxury yacht were found to be heavily corroded a few days after its initial sea trial. The grade of stainless steel was analysed using a hand-held portable X-ray fluorescence machine and found to be correct (grade 316, often referred to as a ‘marine grade’) and typical for the application. However, to offer some ‘protection’ of the rails during the commissioning work, polythene wraps on the rails had been left on. Sea water had been trapped between the rails and polythene, which created crevices.
The sea water could not dry out and the chloride ions inherent in the water readily attacked the stainless steel surface; oxygen availability was limited which prevent repassivation and crevice corrosion developed.
Following discussions with the client, and based on a cost-benefit analysis, the client decided to lightly grind and polish the rails to remove the corrosion damage rather than their replacement. It was advised that inspections be carried out of these surfaces to ensure all remnants of corrosion damage was eliminated otherwise corrosion could continue, but in the form of pitting corrosion. To date, no further damage has been reported.
Example Two
An assembly fabricated from low carbon steel tubes, used in an automotive application, fractured after some months in service. Fracture had occurred at the toe of one of the welds and on initial examination, exhibited evidence of fatigue crack growth which in an automotive application was not unexpected. The area of crack initiation was identified to coincide with a weld stop-start position, and which also exhibited a small area of excess weld deposit which had not fused to the tube (image below).

Cross-section through the area of crack initiation
The unfused area between the weld deposit and the tube created a crevice. On detailed examination of a cross-section through the area of initiation, corrosion in the crevice was observed but also some fine branched cracks that were typical of stress corrosion cracking (SCC). This is an unusual mechanism for low carbon steels but following conversations with the client, it was apparent that prior to painting, the fabrication was subjected to various caustic cleaning processes and this crevice would have harboured remnants of the cleaning chemicals. Combined with residual stress from welding and heating during the drying and baking to cure the paint, this was sufficient to have caused caustic SCC. Fatigue cracking had subsequently developed in service, leading to the failure.
Example Three
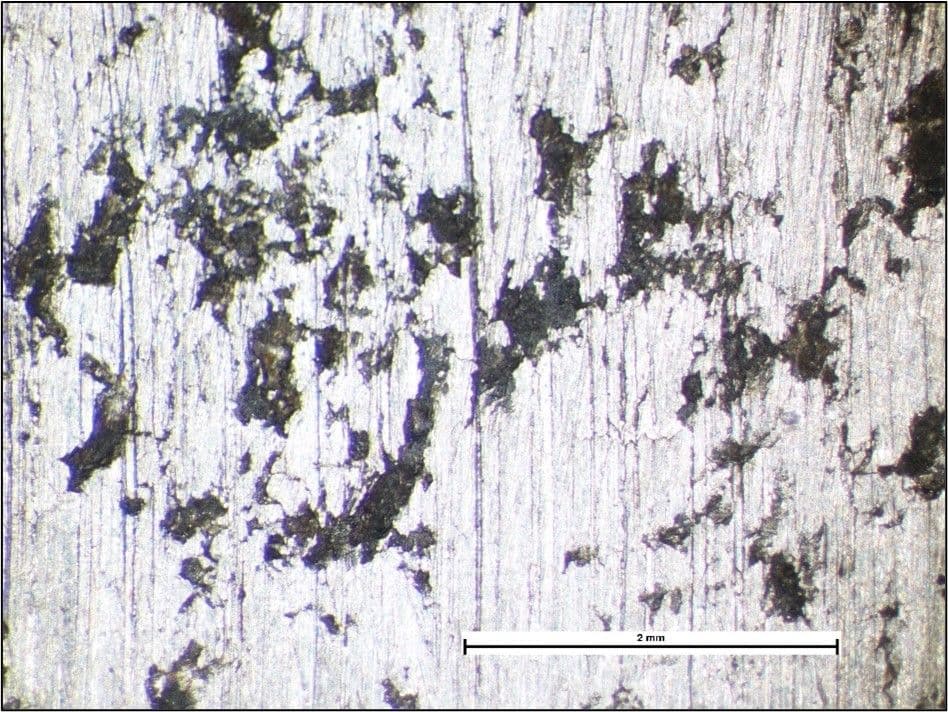
A facility that processed vegetable matter utilised a grade 316 stainless steel structure that consisted of two cylindrical components bonded together with an adhesive. Routine inspection identified corrosion staining on the outer surface of one of the components as shown in the image below. Staining highlighted relatively straight cracks.

Corrosion staining and cracks on the outer surface of a component.
The components were disassembled, and examination of the surfaces bonded with the adhesive revealed crevice corrosion and cracking associated with the corrosion damage shown in the image below.

Investigation identified that edges of the adhesive had debonded from the stainless steel components creating a crevice. The components were routinely exposed to cleaning chemicals that, once concentrated within the crevice, had caused crevice corrosion. However, similar to Example 2 above, then led to SCC which extended for the full thickness of the material.
